Genome Modification Technologies
B-3. Improvement of DNA Break Domains and Development of Single-Base Replacement Platforms
Improvement of DNA cleavage domain and development of single-base-pair editing technology
T. Yamamoto, Hiroshima Univ.
tybighiroshima-u.ac.jp
Technical Description
We developed “FirmCut nuclease” (patent applied for), a novel nuclease possessing higher functionality such as higher cleavage activity and higher flexibility to various target sequences compared to FokI, which is commonly used for the DNA cleavage domain.
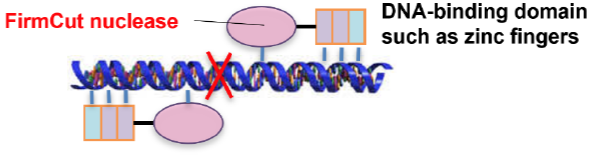
High flexibility (top) and high cleavage activity (bottom) of FirmCut nuclease
ND1, one of the FirmCut nucleases, retains its activity even when the spacer lengths are 5 or 7 bp (red arrows).
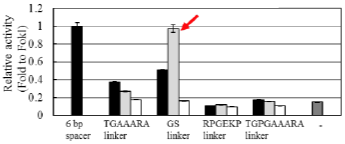
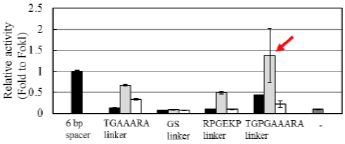
Enhancement of genome editing activity by ND1 and ND2
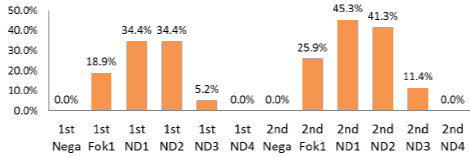
Application Destination
- Enhancement of the functionality of FokI-based genome editing tool such as Platinum TALEN
- Gene modification using a protein-based tool
- Application to made-in-Japan genome editing modules such as PPR
Reference Material
- Polypeptide containing DNA-binding domain (2015-33365)
- Novel nuclease domain and uses thereof (PCT/JP2019/033045)
Contact Information
Takashi YAMAMOTO, Hiroshima University
- E-mail: tybighiroshima-u.ac.jp
- URL: http://www.mls.sci.hiroshima-u.ac.jp/smg/index.html